Why Is Fluorine a Better Oxidizer Than Chlorine
Similarly bromine is a more powerful oxidizing agent than iodine. Fluorine F is the strongest oxidizing agent of all the elements and the other Halogens are also powerful oxidizing agents.

The Most Powerful Oxidising Agent Of The Following Is
Due to its small size Fluorines hydration energy is very high hence better the oxidizing power.

. Fluorine Fluorine having the largest positive value of electrode potential is the. Fluorine is more electronegative than chlorine therefore it can attract a shared pair of electron more easily and strongly than chlorine. Fluorine is such a strong oxidizing agent that it is reasonably impossible to do solution reactions with it.
Bromine can oxidise only iodide ion. Thus it can easily accept the pair of electrons and undergoes reduction. Despite having low electron gain enthalpy it is the stronger oxidising agent than chlorine because of the high hydration energy and low bond dissociation energy.
Thus fluorine is a much stronger oxidizing agent than. Fluorine is the best oxidising agent because it has more reduction potential more ability to lose the electrons. The thing that makes fluorine so reactive is its electronegativity.
Fluorine is a smaller atom than chlorine so the effective pull of its nucleus is greater. Reasonr fluorine is more electronegative than chlorine - Get the answer to this question and access a vast question bank that is tailored for students. One might suppose that the F 9 ion a bare fluorine nucleus would be the best oxidizer and I imagine it would be much better than F 2 but if youre just interested in how good it is at stealing electrons from things in the short term then charge matters much more than the electronegativity of the neutral atom so a bigger nucleus would probably be a better oxidizing.
Among halogens fluorine is the strongest oxidising agent. This is because halogens C l 2 F 2 are good oxidising agents ie their reactivity depend upon their ability to take up an electron and not to give any electron. Fluorine has low bond dissociation enthalpy and high hydration enthalpy due to which fluorine is a good oxidizing agent.
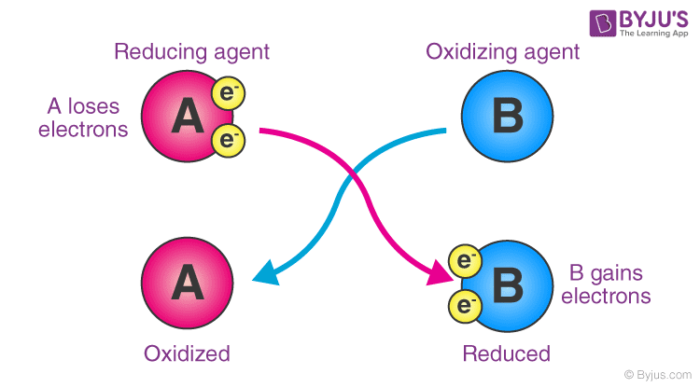
And yes Chlorine has a higher electron affinity than Fluorine. Is fluorine an oxidizing agent. F2 is a stronger oxidizing agent than cl2.
Fluorine is such a good oxidizing agent that metals quartz asbestos and even water burst into flame in its presence. If both assertion and reason are true and reason is the correct explanation of assertion. Correspondingly why is fluorine best oxidizing agent.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Reactivity is an elements ability to gain an electron. While chlorine can oxidise bromine and iodine only.
It is due to its low heat of dissociation and high heat of hydration which have more than compensated the lower value of electron affinity. Fluorine is more powerful oxidising agent than chlorine. Therefore since fluorine has a higher electronegatvity than.
Thus it is a strong oxidizing agent than chlorine. It readily accepts electrons. Fluorine is more oxidising because it is more electronegative than cl so that it can attract a shared pair of e- more easiky and strongly than cl it.
Fluorine is a stronger oxidizing agent than iodine. Why fluorine is stronger oxidising agent than chlorine. Answer 1 of 10.
The electronegativity value of fluorine is 398 according to. P - block element. It is mainly because of the following two reasons 1 due to low bond dissociation.
Chlorine has the ability to take electrons from both bromide ions and iodide ions. Ii High hydration enthalpy of F. Low enthalpy of dissociation of F-F bond 1588 kJ mol-1.
Although electron gain enthalpy of fluorine is less negative as compared to chlorine fluorine is a stronger oxidizing agent than chlorine because of. I found another source stating that bond dissociation enthalpy is the major driving factor. Oxidising agents gain electrons.
That is because although fluorine wants to attract electrons in a bonded pair more easily than chlorine it is more electronegative once it has the electron all to itself gains an electron the repulsive forces in the small size of the fluoride ion comes into effect. Fluorine is achieving octet so electron gain is favorable. Although electron gain enthalpy of fluorine is less negative as compared to chlorine fluorine is a stronger oxidizing agent than chlorine because of.
The tendency of losing electrons attributed to florine due to more electronegativity of it. However the bond dissociation energy of fluorine is much lesser than that of chlorine. It has highest value of oxidation potential.
Due to its small size Fluorines hydration energy is very high hence better the oxidizing power. Low enthalpy of dissociation of F-F bond 1588 kJ mol-1. I Low enthalpy of dissociation of FF bond.
So the latter two factors more than compensate for the less negative electron gain enthalpy of fluorine. So the better it is at stealing electrons the more reactive it will be. In addition its small size the hydration energy of fluorine is much higher than that of chlorine.
If both assertion and reason are true but reason is not the correct explanation of assertion. Greater reactivity of F 2 than C I 2 in aqueous solutions is not due to ionisation potential. Oxidizing agents are substances that gain electrons in a chemical reaction Fluorine is a stronger oxidising agent than chlorine because.
Please log in or register to add a comment. A heat of dissociation b ionisation potential c heat of hydration d electron affinity. Asked Apr 9 2018 in Chemistry by Golu 106k points Fluorine is a stronger oxidising agent than chlorine in aqueous solution.
Fluorine is such a powerful oxidizing agent that solution reactions are. Fluorine has greater electronegativity than iodine. This is attributed to many factors except.
Fluorine has the tendency to oxidise all other halogens in its solid phase. Because of its small size and higher electronegativity than chlorine it is stronger oxidising agent. My questionsalong with reasons why fluorine shouldnt be oxidising agent.
If assertion is true but reason is false. What is the best oxidizing agent.

Is The Oxidizing Power Of Nitrogen Greater Than Flourine Quora

How Is Bromine Synthesised By Laboratory Process Science Chemistry Medicinal Chemistry Chemistry Scientists

Chlorine An Overview Sciencedirect Topics
Which Is More Reactive Among Chlorine And Fluorine Quora

Justify Giving Reactions That Among Halogens Fluorine Is The Best Oxidant And Among Hydrohalic Compounds Hydroiodic Acid Is The Best Reductant

Why Is Fluorine More Oxidizing Than Chlorine Quora

Why Fluorine Is More Oxidizing Than Chlorine P Block Class 12 Youtube

Residential Use Of Hydrogen Peroxide For Treating Iron And Hydrogen Sulfide Wcp Online

Is Chlorine Gas A Stronger Oxidizing Agent Than Oxygen Gas Quora
Is The Oxidizing Power Of Nitrogen Greater Than Flourine Quora

Why Fluorine Is More Oxidizing Than Chlorine P Block Class 12 Youtube

Why Fluorine Is More Oxidizing Than Chlorine P Block Class 12 Youtube
Is Chlorine Gas A Stronger Oxidizing Agent Than Oxygen Gas Quora

Why Fluorine Is More Oxidizing Than Chlorine P Block Class 12 Youtube

Fluorine Is A Strong Oxidising Agent Than Chlorine Even Though Its Negative Electron Gain Enthalpy Is Less Than Chlorine Explain

Uses Of Chlorine And Hydrochloric Acid Infinity Learn
Why Isn T Hydrogen Fluoride A Strong Acid While Fluorine Has The Highest Electronegativity Quora

The Stupidly Dangerous Chemical Chlorine Trifluoride Can Make Anything Burst Into Flames On Contact Latest Science News And Articles Discovery
Comments
Post a Comment